PLEASE MATCH YOUR ASSIGNMENT QUESTIONS ACCORDING TO YOUR SESSION
IGNOU MST-19 (June 2024 – June 2025) Assignment Questions
1. (a) In the natural history of a disease define: total preclinical phase, detectable pre-clinical phase and clinical phase. Suppose on 10 am on 25.01.2010 a disease A onset you biologically. Suppose test of the disease A can detect it exactly after completion of 1000 days of biologically onset. Suppose signs and symptoms develop exactly after completion of 1010 days of biologically onset. Suppose you consult doctor exactly after 1015 days of biologically onset of the disease. Suppose outcome the treatment is cure. What is duration of (i) total preclinical phase (ii) detectable pre-clinical phase (iii) clinical phase.
(b) If p denotes proportion and q denotes odds then prove that q= p/1−P Find the range of q. If the odds of smokers in a study are 0.25 then find the proportion of smokers in the study. Assume that each subject of the study is either a smoker or non-smoker.
(c) A trial is conducted in which some people with disease X were randomly allocated into two groups. First group was advised to do some morning walk for 30 minutes and take light food each day and second group was given one injection and one 100 mg tablet once a day to control disease X. The injection can cause loose motion in some cases and 100 mg tablet has no side effect. At the end of two months, 90% of group I and 80% of group II had recovered from disease X.
i) What are the regimens for group I and group II in this trial?
ii) What are the efficacies in group I and group II?
iii) What are the safety issues in group I and group II in this trial?
2. (a) What is the basic difference between (a) cross-sectional (b) cohort and (c) case control study designs. Explain with suitable examples of each design.
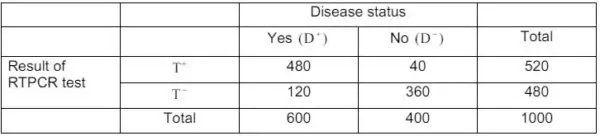
(b) RTPCR test is applied on 300 covid patients and 200 non-covid patients. The results of the test are shown as follows.
What are the sensitivity and specificity of the test? Also, determine the positive and negative values of the test.
3. (a) In usual notations you are given the following information
Find the sample size. If power of the test is 95% instead of 80%, then find new sample size
(b) If you are told that haemoglobin (Hb) level in blood measures the iron content. The normal level in healthy people is around 15 g/dL. Most Indian women have less and some have even less than 8 g/dL. They are called anemic. Iron supplementation is given to increase this level. Suppose one supplementation increase the mean Hb level in anemics by 3.2 g/dL, after one month of use and the other by 3.6 g/dL, in an equivalence trial on 500 women each. The respective SD’s of the of the increases are 0.52 and 0.72 g/dL. The doctors determine that the supplementations can be clinically equivalent if the difference between the increases by two supplementations does not exceed 0.52 g/dL. Can these two supplementations be considered clinically equivalent at 5% level of significance?
IGNOU MST-19 (January 2024 – December 2024) Assignment Questions
1. (a) In the natural history of a disease define: total preclinical phase, detectable pre-clinical phase and clinical phase. Suppose on 10 am on 25.01.2010 a disease A onset you biologically. Suppose test of the disease A can detect it exactly after completion of 1000 days of biologically onset. Suppose signs and symptoms develop exactly after completion of 1010 days of biologically onset. Suppose you consult doctor exactly after 1015 days of biologically onset of the disease. Suppose outcome the treatment is cure. What is duration of (i) total preclinical phase (ii) detectable pre-clinical phase (iii) clinical phase.
(b) If p denotes proportion and q denotes odds then prove that q= p/1−P Find the range of q. If the odds of smokers in a study are 0.25 then find the proportion of smokers in the study. Assume that each subject of the study is either a smoker or non-smoker.
(c) A trial is conducted in which some people with disease X were randomly allocated into two groups. First group was advised to do some morning walk for 30 minutes and take light food each day and second group was given one injection and one 100 mg tablet once a day to control disease X. The injection can cause loose motion in some cases and 100 mg tablet has no side effect. At the end of two months, 90% of group I and 80% of group II had recovered from disease X.
i) What are the regimens for group I and group II in this trial?
ii) What are the efficacies in group I and group II?
iii) What are the safety issues in group I and group II in this trial?
2. (a) What is the basic difference between (a) cross-sectional (b) cohort and (c) case control study designs. Explain with suitable examples of each design.
(b) RTPCR test is applied on 300 covid patients and 200 non-covid patients. The results of the test are shown as follows.
What are the sensitivity and specificity of the test? Also, determine the positive and negative values of the test.
3. (a) In usual notations you are given the following information
Find the sample size. If power of the test is 95% instead of 80%, then find new sample size
(b) If you are told that haemoglobin (Hb) level in blood measures the iron content. The normal level in healthy people is around 15 g/dL. Most Indian women have less and some have even less than 8 g/dL. They are called anemic. Iron supplementation is given to increase this level. Suppose one supplementation increase the mean Hb level in anemics by 3.2 g/dL, after one month of use and the other by 3.6 g/dL, in an equivalence trial on 500 women each. The respective SD’s of the of the increases are 0.52 and 0.72 g/dL. The doctors determine that the supplementations can be clinically equivalent if the difference between the increases by two supplementations does not exceed 0.52 g/dL. Can these two supplementations be considered clinically equivalent at 5% level of significance?