1. a) Calculate the ionisation energy of rubidium per atom, if light of wavelength 5.84 x 10-8 m produces electrons with a speed of 2.450 x 106 ms-1.
[Hint: Assume that the threshold frequency refers to the frequency corresponding to the ionisation energy.]
b) Assume that the electron in Li2+ ion is in third orbit. Calculate
i) the radius of the orbit, and
ii) the total energy of the electron
[Hint: Li2+ ion also has atomic spectra similar to hydrogen atom. while applying relevant equations, use Z = 3.]
2. Using steps 1 to 5 given in Sec. 3.7 of Unit 3, draw the Lewis structures of BrF5 and XeF2. Using VSEPR theory, predict their shapes.
3. Calculate the number of normal modes of vibration of BrF5 and XeF2. Draw diagrams to illustrate the symmetric stretching, asymmetric stretching and bending vibrations of XeF2.
4. a) You are provided with pure copper sulphate crystal. Using Beer-Lambert law, how can you determine the concentration of a test solution of copper sulphate? Explain in a detailed way.
b) What is the essential condition for a molecule to be microwave active? Classify the following molecules as microwave active or microwave inactive and state the reason in each case.
NO, Br2, N2O, XeF2
[Hint: N2O is a linear molecule and one of the nitrogen atoms is at the centre.]
5. a) For 1H19F, the lowest wave number absorption line in its rotational spectrum occurs at 41.11 cm-1. Calculate the wave numbers corresponding to its second, third and fourth absorption lines. Explain the term, rotational spacing using these values.
b) i) Based on Subsec. 6.6.3, draw a rough sketch of PM vs. T-1 curves for BF3 and NH3.
ii) Which of the two, BF3 and NH3, has a nonzero value for orientation polarization? State the reason.
6. Explain the following terms:
a) Moderator
b) Breeder reactor
c) Nuclear fusion
d) Transmutation reaction
e) Tracer technique
7. a) Draw the enantiomers for 2, 3-dibromopentane. Identify at least one pair of diastereomers among these structures. Can it form meso form? State the reason for your answer.
b) Explain one application each for the study of paramagnetic and diamagnetic substances sing magnetic susceptibility measurements. Comment on their dependence of these values of temperature.
8. a) State the modification that was needed for Bohr’s atom model in view of Heisenberg’s uncertainty principle.
b) Explain the need to introduce normalization constant and, complex conjugate of wave function.
c) Using Table 2.2, and the equation given in Eq. 2.54 of Unit 2, explain the directorial characteristics of 2px and 3dxz orbitals of a species with one electron.
9. a) On the basis of molecular orbital theory, draw the energy pattern for
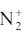
ion. Comment on the bond order. Is it paramagnetic? Explain.
b)
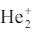
ion has less stable arrangement that H2 molecule. Explain using molecular orbital theory.
c) Explain with an example, the term, nonbonding molecular orbitals.
10. a) In the following compound, indicate the type of hybridization of each carbon atom. Also predict the carbon-carbon bond lengths using Table 4.4.
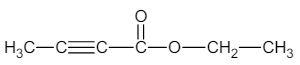
b) Explain, using hybridization theory and diagram, the structure of allene, CH2 = C = CH2.
1. a) Calculate the energy values of the electron in second and third orbits of hydrogen atom.
b) Calculate the ionisation energy of hydrogen atom using Bohr’s theory.
c) For the first two lines of Balmer series, identify the values of n1 and n2.
2. a) The first ionisation energies of silicon and sulphur are lower than that of phosphorus. Explain.
b) Explain use of cation to anion radius ratio.
c) Arrive at the Lewis structures of
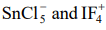
using the steps indicated in Sec. 3.7 of Unit 3.
d) The bond enthalpies of N–N, N=N and N≡N bonds are not in the ratio 1:2:3. Explain the reason.
3. a) Using Pauli’s exclusion principle, work out the total number of electrons in the level n=3.
b) For a particle in one-dimensional box, write the equations for calculating the energy values corresponding to
i) n = 1
ii) n = 2
iii) n = 3
c) Calculate the de Broglie wavelength associated with a ball of mass 0.50 kg moving with a velocity of 20 m s−1.
d) What is a well-behaved wave function?
4. a) State the definitions of bonding, anti-bonding and nonbonding orbitals. Draw the molecular orbitals obtained by the linear combination of two 1s orbitals.
b) Write down the molecular orbital configurations of C2 and N2. Calculate their bond orders. Which of these two is expected to have higher bond energy?
5. a) Assuming that the covalent radius of hydrogen is 28 pm when it is bonded to other atoms, and using Table 3.10, calculate the bond lengths of the bonds in
CH2 = CH−CH2−NH2.
b) Alkyl halides are more reactive than alkanes. Explain the reason.
c)
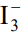
ion is known to exist but not
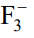
ion. Explain the reason after assigning Lewis structures for both the ions.
6. a) Write down the structures of stereoisomers of 2, 3–pentanediol. Identify the enantiomeric pairs.
b) State the difference between the racemic mixture and meso form.
c) Suggest a method of determination of molar extinction coefficient.
7. a) Write the resonance structures of formate ion. What do you infer regarding the relative bond length values of two C−O bonds in it? Explain the reason.
b) Predict the hybridisation state of each carbon atom in the following compounds:
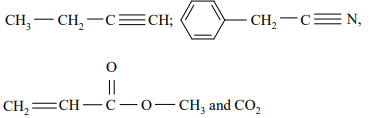
8. a) The rate constant for Cr decay is 2.89×10−7 s−1. Calculate the time required for 87.5% decay.
b) Explain the method of determining the age of an organic material.
c) Explain the following terms:
i) Moderator and
ii) Breeder reactor.
9. a) Among the molecules given below, identify those which are microwave active.
Br2, HF, CO2 and CO
b) Write down the values of two characteristic frequencies for each of the following compounds:
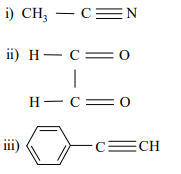
c) State the condition to be satisfied for a molecule to absorb in the microwave region.
10. a) Give an example for each of the following types of nuclear transmutation reactions:
( p, α), ( n, p), ( n, α) and ( p, n)
b) An analyst is asked to oxidize a secondary alcohol to ketone. What single characteristic feature in IR spectra should the analyst look for in order to verify the feasibility of the reaction?
c) Which of the following has higher λmax value?
1-butene or 1, 3 – butadiene.
State the reason.
d) Calculate the molar diamagnetic susceptibility value of acetophenone.

 b) Explain, using hybridization theory and diagram, the structure of allene, CH2 = C = CH2.
b) Explain, using hybridization theory and diagram, the structure of allene, CH2 = C = CH2.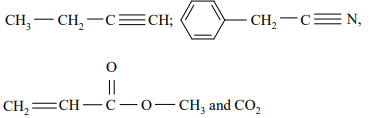 8. a) The rate constant for Cr decay is 2.89×10−7 s−1. Calculate the time required for 87.5% decay.
b) Explain the method of determining the age of an organic material.
c) Explain the following terms:
i) Moderator and
ii) Breeder reactor.
9. a) Among the molecules given below, identify those which are microwave active.
Br2, HF, CO2 and CO
b) Write down the values of two characteristic frequencies for each of the following compounds:
8. a) The rate constant for Cr decay is 2.89×10−7 s−1. Calculate the time required for 87.5% decay.
b) Explain the method of determining the age of an organic material.
c) Explain the following terms:
i) Moderator and
ii) Breeder reactor.
9. a) Among the molecules given below, identify those which are microwave active.
Br2, HF, CO2 and CO
b) Write down the values of two characteristic frequencies for each of the following compounds:
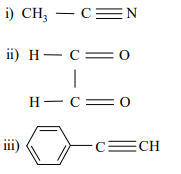 c) State the condition to be satisfied for a molecule to absorb in the microwave region.
10. a) Give an example for each of the following types of nuclear transmutation reactions:
( p, α), ( n, p), ( n, α) and ( p, n)
b) An analyst is asked to oxidize a secondary alcohol to ketone. What single characteristic feature in IR spectra should the analyst look for in order to verify the feasibility of the reaction?
c) Which of the following has higher λmax value?
1-butene or 1, 3 – butadiene.
State the reason.
d) Calculate the molar diamagnetic susceptibility value of acetophenone.
c) State the condition to be satisfied for a molecule to absorb in the microwave region.
10. a) Give an example for each of the following types of nuclear transmutation reactions:
( p, α), ( n, p), ( n, α) and ( p, n)
b) An analyst is asked to oxidize a secondary alcohol to ketone. What single characteristic feature in IR spectra should the analyst look for in order to verify the feasibility of the reaction?
c) Which of the following has higher λmax value?
1-butene or 1, 3 – butadiene.
State the reason.
d) Calculate the molar diamagnetic susceptibility value of acetophenone.