
#1 Best Selling IGNOU Assignments in All Available in Market
Bought By: 4678 Students
Rating: 4.3
Get IGNOU CHE-10 Assignments Soft Copy ready for Download in PDF for (January 2024 - December 2024) in English Language.
Are you looking to download a PDF soft copy of the Solved Assignment CHE-10 - Spectroscopy? Then GullyBaba is the right place for you. We have the Assignment available in English language.
This particular Assignment references the syllabus chosen for the subject of Chemistry, for the January 2024 - December 2024 session. The code for the assignment is CHE-10 and it is often used by students who are enrolled in the B.Sc. Degree.
Once students have paid for the Assignment, they can Instantly Download to their PC, Laptop or Mobile Devices in soft copy as a PDF format. After studying the contents of this Assignment, students will have a better grasp of the subject and will be able to prepare for their upcoming tests.
1. Draw a labeled diagram for various series of spectral lines observed in the atomic spectrum of hydrogen atom.
2. Derive the spectroscopic states of carbon atom.
3. Illustrate the axis/axes of symmetry present in the following molecules:
(i) H2O (ii) NH3 (iii) BF3 (iv) benzene
4. Which elements of symmetry are present in the following molecules?
(i) PCl5 (ii) C2H2
Also give the point groups of these molecules.
5. Show transitions between various rotational levels and spectral lines arising from such transitions for a rigid diatomic molecule. What is the difference between various transitions?
6. (a) Explain the following terms:
(i) Zero point energy
(ii) Fundamental transitions
(iii) First overtone
(b) What is Morse potential? Briefly discuss.
7. Discuss the IR spectrum exhibited by water molecule. Also draw the suitable diagram.
8. Explain the following:
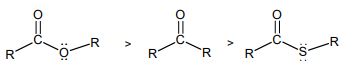
(i) Benzophenone absorbs at 1700 cm-1 while acetone absorbs at 1720 cm-1 in their IR spectra.
(ii) The IR absorptions frequencies of the following compounds are in the order shown below:
9. How do the stokes, anti-stokes and Rayleigh lines appear in the Raman spectrum? Explain giving the suitable diagram.
10. Explain the IR bands exhibited by CO2 and SO2 in the light of mutual exclusion principle and arrive at the structures of these molecules.
11. Define the following terms:
(i) Auxochrome
(ii) Chromophone
(iii) Hypsochromic shift
(iv) Hypochromic effect
(v) Hyperchromic effect
12. Discuss the effect of change of solvent from polar to non-polar on the π→π∗ and n→π∗ transitions.
13. Draw the crystal field splitting of d-orbitals of metal ions in complexes having different geometries.
14. Briefly explain fluorescence using a suitable diagram.
15. Draw the back diagram of a single beam IR spectrometer. How does a double beam instrument different from that of the single beam IR spectrometer?
16. Show that the energy difference between two spin states of a nucleus is given by
ΔE =| gN |BNBZ
17. Draw and explain the N,M,R spectrum of CH3CHO molecule.
18. Draw and explain the functioning of an ESR spectrometer.
19. What is simple cleavage? Illustrate it with the fragmentation of a suitable molecule.
20. Explain spectral signals expected in the different spectra of benzyl alcohol. What different units of the molecules are responsible for them?
1. Discuss the vector nature of angular momentum giving a suitable diagram.
2. What is Rydberg constant? Show that its value is 1.09737 x 10 7 m-1.
3. Illustrate improper axis of rotation giving a suitable example.
4. Write the symmetry elements and point groups of the following molecules:
(i) CH4 (ii) SF6 (iii) BF3
5. For NO molecule, the rotational constant, B is 1.70 cm-1. Calculate its bond length.
6. Explain the following:
(i) A Harmonic oscillator
(ii) Hooke’s law
7. Discuss the IR spectrum exhibited by CO2 molecule giving the suitable diagram.
8. Which absorptions of different molecules appear in the 1500-600 cm-1 region of IR spectra? Explain.
9. What are the selection rules for rotational Raman spectra of linear molecules? Draw a Schematic diagram for pure rotational Raman spectrum of a diatomic molecule.
10. Explain the shape of ![]() ion using its IR and Raman spectra.
ion using its IR and Raman spectra.
11. Derive the term symbols for O2 molecule.
12. Discus the effect of change of polarity of the solvent on the following transitions:
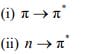
Draw a suitable diagram.
13. Explain giving a suitable diagram, the crystal field splitting of orbitals of metal ion in complexes having different geometries.
14. Explain the following terms:
(i) Vibrational relaxation
(ii) Fluorescence and phosphorescene quenching.
15. (a) Answer the following in two to three sentences:
(i) Glass container cannot be used for IR spectroscopy?
(ii) What are the basic requirements of any container which holds the sample?
(iii) What is the significance of monochromators?
(b) Draw the block diagram for the following:
(i) Raman spectrometer
(ii) IR spectrometer
16. (a) What is nuclear magneton? Calculate its value for a proton.
(b) Give two examples each for nucleic having
I = 0 and I = 1
17. Explain chemical shift. How it is expressed?
18. Discuss the esr spectrum of hydrogen atom giving a suitable diagram.
19. Discuss the mass spectrum of 2-methylpentane giving the fragments formed.
20. What would be different signals observed in the spectra of 4-ketobutanoic acid? Explain which structural units are responsible for these spectral signals.
The IGNOU open learning format requires students to submit study Assignments. Here is the final end date of the submission of this particular assignment according to the university calendar.
Here are the PDF files that you can Download for this Assignment. You can pick the language of your choice and see other relevant information such as the Session, File Size and Format.
In this section you can find other relevant information related to the Assignment you are looking at. It will give you an idea of what to expect when downloading a PDF soft copy from GullyBaba.
In addition to this Assignment, there are also other Assignments related to the B.Sc. Chemistry you are preparing for. Here we have listed other Assignments that were bought along with this one.