
#1 Best Selling IGNOU Assignments in All Available in Market
Bought By: 5652 Students
Rating: 4.2
Get IGNOU PHE-06/BPHE-106 Assignments Soft Copy ready for Download in PDF for (January 2024 - December 2024) in English Language.
Are you looking to download a PDF soft copy of the Solved Assignment PHE-06/BPHE-106 - Thermodynamics and Statistical Mechanics? Then GullyBaba is the right place for you. We have the Assignment available in English language.
This particular Assignment references the syllabus chosen for the subject of Physics, for the January 2024 - December 2024 session. The code for the assignment is PHE-06/BPHE-106 and it is often used by students who are enrolled in the B.Sc. Degree.
Once students have paid for the Assignment, they can Instantly Download to their PC, Laptop or Mobile Devices in soft copy as a PDF format. After studying the contents of this Assignment, students will have a better grasp of the subject and will be able to prepare for their upcoming tests.
1. Answer any four parts.
a) A block of metal whose volume expansivity is 5.0 × 10–5 °C -1 and isothermal compressibility is 1.2 × 10–6 atm-1 has volume 5 litre at 1 atm and 20°C. On applying pressure, its temperature rises by 10°C and volume increases by 0.5 cm3 . Calculate the pressure applied.
b) Define adiabatic lapse rate? Obtain an expression for adiabatic lapse rate for earth’s atmosphere?
c) A Carnot engine has an efficiency of 40%. Its efficiency is to be raised to 50%. By how much must the temperature of the source be increased if the sink is at 27°C.
d) The mean speed of oxygen molecules is 450 ms–1 . If the radius of an oxygen molecule is 1.8 Å, calculate mean time between two successive collisions and mean free path. Take n= 3 × 10 25 m–3.
e) Write an expression for the partition function for an ideal gas made up of N indistinguishable particles. Using this expression, obtain Sackur-Tetrode equation.
2. a) A reversible process can only be idealised and cannot be achieved in practice. Justify.
b) Explain the working of a platinum resistance thermometer with the help of a neat and labelled diagram. State the reasons for using platinum for the construction of a resistance thermometer.
c) Write the expressions for the work done for (i) paramagnetic substance, and (ii) stretched wire. Calculate the work done on the steel wire of length 2.5 m and area of cross-section 2.5 × 10–6 m 2 is suspended from torsion head when a 5 kg weight is suspended at its free end. Take Y = 2 × 1011 Nm–2 .
3. a) Explain the statement “entropy of earth-sun system increases” in the light of the principle of increase of entropy.
b) Draw Carnot cycle on (i) p-V, and (ii) T-S diagrams. With the help of p-V diagram, show that , Q1/Q2=T1/T2, where Q1 is the amount of heat absorbed at temperature T1 and Q2 is the amount of heat rejected at temperature T2 .
c) Using Maxwell’s relations, prove second energy equation:
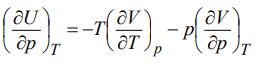
Discuss its physical significance
4. a) State the principle of equipartition of energy. Write a relation between number of degrees of freedom, number of particles constituting the system and the total number of constraints. Calculate the degrees of freedom for (i) single atom, (ii) diatomic molecule.
b) Derive Einstein’s formula for mean square displacement of a Brownian particle.
c) Calculate the coefficient of viscosity of hydrogen at STP. Take
ρ = 8.90 × 10–2 kg m–3, λ = 2 × 10–7 m and kB = 1.38 × 10 –23 JK–1.
5. a) Establish Boltzmann relation between entropy (S), and thermodynamic probability (W):
S = kB ln W.
b) What is a Gibbs paradox? How did it arise?
c) Using the expression of Bose-Einstein distribution function for photons
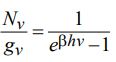
derive Plancks Law and show that (i) Rayleigh-Jeans Law, and (ii) Wien’s Law follow from it at low & high frequencies.
1. a) Define degree of freedom of a molecule. Write the formula for the number of degrees of freedom of a molecule. Calculate the degree of freedom for a rigid diatomic molecule.
b) The average energy of a helium molecules is 2.89 x 10 -21 J. Calculate their most probable speed and average speed.
c) Write the van der Waals’ equation of state. Using this equation, obtain the critical constants and show that
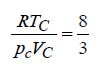
d) Derive an expression for survival equation for distribution of free paths. Also, plot survival equation.
e) Derive Einstein formula for mean square displacement of a Brownian particle.
2. a) Describe the construction and working of a Platinum resistance thermometer. Write its two principal merits.
b) Define the followings with example: (i) Intensive variables (ii) Extensive variables (iii) Adiabatic boundary (iv) Open system (v) Isolated system.
c) Obtain the expression for isothermal compressibility (βT) and coefficient of volume expansion (α) for a van der Waals’ gas.
d) Obtain an expression for work done by an ideal gas in an adiabatic process. Two litre of an ideal gas at a pressure of 5 atm expands adiabatically to two times its initial volume. Calculate the work done by the gas. Given ϒ= 1.4.
e) Derive an expression for Clausius- Clayperon equation.
3. a) Draw a T-S diagram of a Carnot cycle and derive an expression of efficiency of a heat engine working between T1 and T2.
b) A freezer operates between –15°C and 27°C. Calculate the maximum value of coefficient of performance (ω) for this refrigerator. With this ω, how much electrical energy would be required to freeze 0.8kg of water, initially at 0°C. Given specific latent heat of fusion = 334 kJ kg -1.
c) Using Maxwell’s relations, derive first and second TdS equations.
d) What is Joule-Thomson effect? Derive an expression of Joule-Thomson coefficient for a van der Waals’ gas.
4. a) The thermodynamic probability for a Boson system is given by
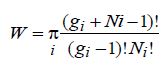
Using this relation, derive an expression for the Bose-Einstein distribution function.
b) Establish the Boltzmann relation S = kB ln W.
c) What is Gibbs paradox? Derive the Sackur-Tetrode equation for the entropy for an ideal monatomic gas.
d) The expression for Planck’s law for energy density is given by
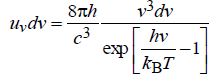
Using this expression, obtain (i) Wien’s law and (ii) Stefan-Boltzmann law.
The IGNOU open learning format requires students to submit study Assignments. Here is the final end date of the submission of this particular assignment according to the university calendar.
Here are the PDF files that you can Download for this Assignment. You can pick the language of your choice and see other relevant information such as the Session, File Size and Format.
In this section you can find other relevant information related to the Assignment you are looking at. It will give you an idea of what to expect when downloading a PDF soft copy from GullyBaba.
In addition to this Assignment, there are also other Assignments related to the B.Sc. Physics you are preparing for. Here we have listed other Assignments that were bought along with this one.