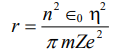PLEASE MATCH YOUR ASSIGNMENT QUESTIONS ACCORDING TO YOUR SESSION
IGNOU BCHCT-131 (January 2025 – December 2025) Assignment Questions
IGNOU BCHCT-131 (January 2024 – December 2024) Assignment Questions
PART-(A)
1. Using a suitable diagram explain the spectral transitions between different energy levels of hydrogen atom. Also name these series of lines and give the region of electromagnetic radiation in which they appear.
2. What was the purpose of Davisson and Germer experiment? Explains and analyse its results.
3. (a) What is a well-behaved wave function? Illustrate using suitable diagram.
(b) Give the significance of ψ and ψ2.
4. What are different quantum numbers? Explain their significance.
5. Briefly explain the following:
(i) The aufbau principle
(ii) Hund’s rule
(iii) Pauli exclusion principle
6. a) Arrange the following compounds in order of decreasing lattice energy: LiF,MgO,KBr. Justify your answer.
b) Predict coordination number of the cation in crystals of the following compounds.
MgO: if ionic radii for Mg2+ =65 pm and O2– = 140 pm.
MgS: if ionic radii for Mg2+ =65 pm and O2– = 184 pm.
7. Why does the bond length decrease in the case of multiple bond formation? Explain with the help of an example. Also explain why a multiple bond is stronger than a single bond.
8. a) The observed dipole moment of HI is 0.38 D. Calculate the percentage ionic character of the bonding HI if bond distance is 161 pm
b) Melting point of aluminum fluoride is higher than the melting point of aluminum iodide. Explain
9. Draw the resonance structures of carbon monoxide. Also give the electronic configuration of the combining atoms
10. Draw the energy level diagram for carbon monoxide molecule. Write its molecular orbitals configuration and calculate its bond order. Comment on its magnetic behaviour.
PART-(B)
11. Draw all the stereoisomers of 2-bromo-3-chlorobutane and classify them as enantiomers and diastereoisomers.
12. (a) What are resolving agents? Give examples of three acidic and three basic resolving agents.
(b) Write the Fischer projection for the molecule.
13. Draw and explain the energy propile for ring flipping of chair conformation of cyclohexane.
14. Arrange the following carbocations in the increasing order of stability and explain the reason for your answer:
A primary carbocation, a tertiary carbocation, a secondary carbocation.
15. Arrange the following nucleophiles in the increasing order of their strength and give reason for your answer.
CH3-, NH2-, CN-, OH-, I-
16. (a) Define octane number. How does the octane number of a hydrocarbon vary with the following?
(i) Branching of the hydrocarbon chain
(ii) Decrease in the chain length
(iii) Unsaturation
(b) How would you synthesise hexane using Wurtz reaction. Explain giving equation.
17. How would you prepare an alkene using Wittig reaction? Explain the mechanism also.
18. What is Markownikoff’s rule? Explain using this rule why 2-bromopropane is the major product of bromination of propene.
19. Discuss different methods of preparation of propyne.
20. Explain whether the following compounds are aromatic or not?