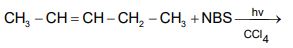PLEASE MATCH YOUR ASSIGNMENT QUESTIONS ACCORDING TO YOUR SESSION
IGNOU MCH-12 (January 2025 – December 2025) Assignment Questions
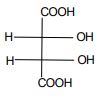
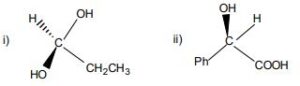
1. Carry out the following conversions as indicated.
a) Fischer form to: i) Staggered and Eclipsed Sawhorse forms and
ii) Staggered and Eclipsed
b) Flying wedge form to Fischer forms
2. a) Suppose that there is a mixture having a specific rotation of +75o and an enantiomeric excess of 60%. Find out the enantiomeric composition of the mixture.
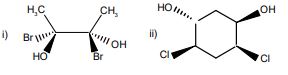
b) Draw the meso isomers of the following compounds
3. a) List the properties of diastereomers. Indicate whether the following are enantiomers or diastereomers
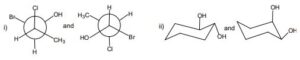
b) How are disubstituted cyclohexanes different from monosubstituted cyclohexanes with reference to the isomerism? Draw the two chair conformations for each of the following disubstituted cyclohexanes. Draw also the cis and trans isomers and indicate the more stable isomer.
i) cis-Bromo-1-methylcyclohexane
ii) trans-2-Butyl-1-isopropylcyclohexane
iii) cis-4-Ethyl-1-hydroxycyclohexane
4. a) What are the advantages and limitations of method of quasi-racemates?
b) What is pseudoasymmetry? Explain with the help of a suitable example.
5. a) Briefly explain conformationally rigid systems and conformationally mobile diostereomers giving one example for each. Also correlate the reactivity and the conformations of these examples.
b) What are left- and right- circularly polarised lights? Draw suitable diagrams for them.
6. a) Give any two applications of ORD and CD.
b) What is the principle of microscopic reversibility? Explain taking the example of an addition-elimination reaction or a nucleophilic substitution reaction.
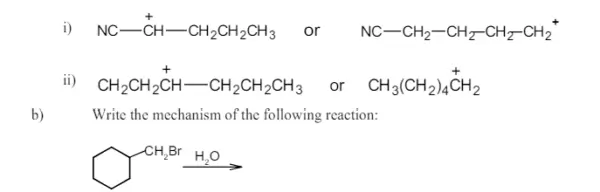
7. a) Select the resonance stabilised cation and give reason for your answer.
b) Write the product formed and give the mechanism.
8. a) Does a carbanion behave as an acid or a base? Explain the stability of carbanion on the basis of this behaviour?
b) Arrange the following anions in the decreasing order of stability
9. a) Predict the product and write the mechanism
b) Arrange the following radicals in their increase order of stability and give the reason of your answer
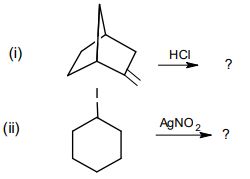
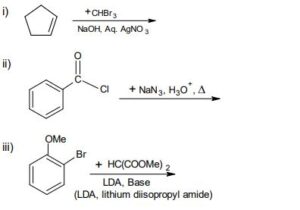
10. Write the products formed in each of the following and give the mechanism.
IGNOU MCH-12 (January 2024 – December 2024) Assignment Questions
1. a) Name the two ways of representing the three dimensional structures of molecules in two dimensions and explain the types involved any one of these. Illustrate your answer.
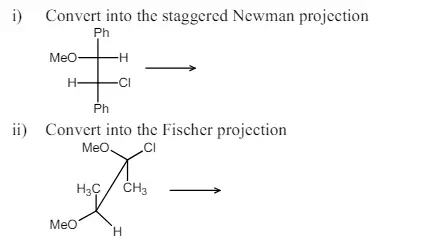
b) Carry out the following conversions for the structures given indicating the projection of the molecule.
i) Convert into the staggered Newman projection
2. a) Explain the stability of methylcyclohexane with the help of chair conformations and the Newman projections.
b) Write the order of simple axis of symmetry and the plane or symmetry in the following molecules.
3. a) How is the asymmetry observed in allenes or spiranes different from that observed in biphenyls? Explain giving example in both the cases.
b) Explain the method of quasi-racemates for the determination of configuration giving suitable diagrams.
4. a) i) Explain the Re and Si faces in CH3CHO using suitable diagrams.
ii) What is a stereocentre? Does the presence of stereocentres ensure chirality in a compound?
b) i) What is chirotopicity and a chirotopic atom? (2)
ii) Explain the presence of chirotopic atoms in a chiral molecule and an achiral molecule.
5. a) Illustrate Cram’s rule by using open chain model by taking suitable examples.
b) Explain the octant rule using suitable diagram and give rules used for contribution of different substituents.
6. a) Draw the potential energy diagrams (PED) for the following reaction indicating:
i) Step 1 as the rate determining step
ii) Step 2 as the rate determining step
b) How are stereochemical studies helpful in establishing the mechanism of organic reactions illustrate your answer.
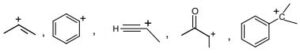
7. a) Which is more stable in the following pairs and why? Indicate the carbocations as primary secondary or tertiary.
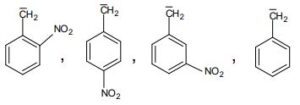
8. a) Arrange the following compounds in the increasing order of their stability. Give reason for your answer.
Allyl anion, m-Nitrobenzyl anion, ortho Chlorobenzyl anion, Benzyl anion
b) Draw the structures of the two types of carbenes. Explain the stereochemistry of addition reaction on these types.
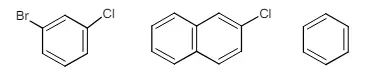
9. a) Give the evidence which explains the formation of benzyne intermediate during nucleophilic substitution reactions of aromatic halides.
b) Why is bromination by free radical mechanism is more selective than chlorination? Write all the possible chlorination products of methyl cyclohexane by photolysis.
10. a) Compare the nature of nitrenes to carbenes and give two methods of generation of nitrenes.
b) Describe the various redox sources of free radical generation.